The Role of Proteoglycans in Bone Structure and Function
When we think of bones, we often imagine them as rigid structures that support our bodies, protect vital organs, and enable movement. While bones are indeed strong and sturdy, they are also dynamic and complex tissues composed of a variety of cells and molecules that work together to maintain their strength and flexibility. To understand bone structure and function, it’s essential to review the key components of bone tissue: the mineralized matrix and the organic matrix. The mineralized matrix is primarily made of hydroxyapatite crystals, a calcium phosphate compound that provides bones with their hardness and strength. The organic matrix, on the other hand, is composed mainly of collagen fibers and proteoglycans. While collagen fibers give bone its tensile strength and resistance to stretching, proteoglycans contribute to the flexibility and resilience of bone.
1. Decorin: Decorin binds to collagen fibers, regulating their organization and assembly. This interaction is essential for maintaining the integrity and strength of the bone matrix. Decorin also modulates the activity of growth factors, such as transforming growth factor-beta (TGF-β), which is involved in bone remodeling and repair.
2. Biglycan: Similar to decorin, biglycan interacts with collagen fibers and influences the mineralization process by regulating the deposition of hydroxyapatite crystals in the bone matrix. It is expressed during cell proliferation and mineralization and has a role in regulating the activity of bone cells, including osteoblasts (bone-forming cells) and osteoclasts (bone resorbing cells).
3. Osteoadherin (Osteomodulin): Osteoadherin is expressed in mineralized tissues, including bones and teeth. It binds to hydroxyapatite crystals, contributing to the mineralization of bone. By interacting with collagen fibers, osteoadherin helps to organize the bone matrix and maintain its structural integrity.
4. Asporin: This unique member of the SLRP family contains a distinctive series of aspartate residues in the N-terminal region, with some polymorphisms linked to osteoarthritis and intervertebral disc disease. In skeletal tissue, asporin promotes collagen mineralization and acts as a negative regulator of chondrogenesis.
5. Keratocan: This keratan sulfate proteoglycan plays a role in regulating cell proliferation and modulation of osteoblast differentiation.
6. Versican: Although not an SLRP, Versican is a large proteoglycan in bone that plays a significant role in bone. It is known for its ability to bind water and contribute to the viscoelastic properties of the extracellular matrix. In bone, versican helps modulate the mechanical properties of the tissue, providing resistance to compressive forces.
Advances in bone tissue engineering have led to the development of biomimetic materials that replicate the function of natural proteoglycans. One such material is a hyper-crosslinked carbohydrate-based polymer (Osteo-P® BGS). This novel polymer displays similar characteristics to those of proteoglycans, being biocompatible, osteoconductive, and promoting significant bone regeneration in vivo.
Osteo-P® BGS scaffolds mimic essential properties of proteoglycans, including strong binding with growth factors and high density, interconnected pores with a large surface area, which facilitate absorption, nutrient transfer, and cell adhesion. Remarkably, these scaffolds have demonstrated exceptional adherence of hematopoietic (CD34+) and mesenchymal stem cells, promoting enhanced osteogenic (bone-forming) and angiogenic (blood vessel-forming) properties.
Understanding the structure and function of proteoglycans in bone biology not only provides insights into how our skeletal system functions, but also opens potential avenues for developing new treatments for bone-related diseases and conditions. As research progresses, materials that mimic the properties of proteoglycans, like Osteo-P® BGS, offer promising strategies for bone regeneration and repair.
For more in-depth reading, refer to “Osteogenic Cells and Microenvironment of Early Bone Development and Clinical Implication” (D. Kim & C. Lee, 2023) and “Hyper-Crosslinked Carbohydrate Polymer for Repair of Critical-Sized Bone Defects” (Koleva et al., 2019).
Chen, J., Sun, T., You, Y., Wu, B., Wang, X., & Wu, J. (2021). Proteoglycans and Glycosaminoglycans in Stem Cell Homeostasis and Bone Tissue Regeneration. Frontiers in Cell and Developmental Biology, 9, 760532. https://doi.org/10.3389/fcell.2021.760532
D. Kim, K., & C. Lee, C. (2023). Osteogenic Cells and Microenvironment of Early Bone Development and Clinical Implication. In J. Jin Wang, G. Wang, X. Lv, Z. Sun, & K. Sunil Mahapure (Eds.), Frontiers in Spinal Neurosurgery. IntechOpen. https://doi.org/10.5772/intechopen.1002037
Filippi, M., Born, G., Chaaban, M., & Scherberich, A. (2020). Natural Polymeric Scaffolds in Bone Regeneration. Frontiers in Bioengineering and Biotechnology, 8, 474. https://doi.org/10.3389/fbioe.2020.00474
Guo, L., Liang, Z., Yang, L., Du, W., Yu, T., Tang, H., Li, C., & Qiu, H. (2021). The role of natural polymers in bone tissue engineering. Journal of Controlled Release, 338, 571–582. https://doi.org/10.1016/j.jconrel.2021.08.055
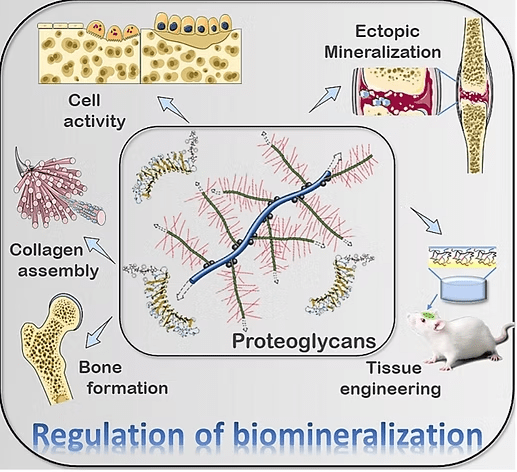
Jia-xin Hao, Min-juan Shen, Chen-yu Wang, Jian-hua Wei, Qian-qian Wan, Yi-fei Zhu, Tao Ye, Meng-lin Luo, Wen-pin Qin, Yu-tao Li, Kai Jiao, Bin Zhao, Li-na Niu. Regulation of biomineralization by proteoglycans: From mechanisms to application. Carbohydrate Polymers, Volume 294, 2022, 119773. https://doi.org/10.1016/j.carbpol.2022.119773
Koleva, P. M., Keefer, J. H., Ayala, A. M., Lorenzo, I., Han, C. E., Pham, K., Ralston, S. E., Kim, K. D., & Lee, C. C. (2019). Hyper-Crosslinked Carbohydrate Polymer for Repair of Critical-Sized Bone Defects. BioResearch Open Access, 8(1), 111–120. https://doi.org/10.1089/biores.2019.0021
Lin, X., Patil, S., Gao, Y.-G., & Qian, A. (2020). The Bone Extracellular Matrix in Bone Formation and Regeneration. Frontiers in Pharmacology, 11, 757. https://doi.org/10.3389/fphar.2020.00757
Robey, P. G. (2002). Bone Matrix Proteoglycans and Glycoproteins. In Principles of Bone Biology (Second, Vol. 1, pp. 225–237). https://doi.org/10.1016/B978-012098652-1.50116-5
Sampaolesi, S., Nicotra, F., & Russo, L. (2019). Glycans in nanomedicine, impact and perspectives. Future Medicinal Chemistry, 11(1), 43–60. https://doi.org/10.4155/fmc-2018-0368
Sivakumar, P. M., Yetisgin, A. A., Sahin, S. B., Demir, E., & Cetinel, S. (2022). Bone tissue engineering: Anionic polysaccharides as promising scaffolds. Carbohydrate Polymers, 283, 119142. https://doi.org/10.1016/j.carbpol.2022.119142
Witzler, M., Büchner, D., Shoushrah, S., Babczyk, P., Baranova, J., Witzleben, S., Tobiasch, E., & Schulze, M. (2019). Polysaccharide-Based Systems for Targeted Stem Cell Differentiation and Bone Regeneration. Biomolecules, 9(12), 840. https://doi.org/10.3390/biom9120840
Jia-xin Hao, Min-juan Shen, Chen-yu Wang, Jian-hua Wei, Qian-qian Wan, Yi-fei Zhu, Tao Ye, Meng-lin Luo, Wen-pin Qin, Yu-tao Li, Kai Jiao, Bin Zhao, Li-na Niu. Regulation of biomineralization by proteoglycans: From mechanisms to application. Carbohydrate Polymers, Volume 294, 2022, 119773. https://doi.org/10.1016/j.carbpol.2022.119773.
© 2025 Molecular Matrix, Inc. All rights reserved.